

Human papillomaviruses (HPVs) are a group of viruses that infect the outer and inner surfaces of the human body and cause a number of health problems.1,2 These surfaces include the skin as well as the mucous membranes (moist tissue that lines the inner surfaces of the body). Each type, or genotype, of HPV tends to infect only a particular body surface area. For example, HPV type 1 (HPV-1) often causes warts on the soles of the feet (plantar warts),3 whereas HPV-2 cause other common warts.3 Other types of HPV can infect the mouth and throat, as well as the genitals and anus. These types of HPV, which cause genital warts, are commonly spread by sexual contact.4
There are more than 200 different types of HPV.2 Each HPV type is assigned a number and classified as low-risk or high-risk. Low-risk types of HPV may cause warts on the cervix and in the vagina or around the genitals and anus of both men and women, but they rarely cause cancer. At least 13 types of HPV are considered high-risk because a long-term infection with those strains can cause cancer5—in particular, cancer of the cervix, vulva, vagina, penis, or anus, as well as the oropharynx (back of the throat).4
The most common high-risk types are HPV-16 and HPV-18.6 In 2008, Harald Zur Hausen won the Nobel Prize for discovering that about 50% of all cervical cancers (tumors of the uterine cervix) were infected by HPV-16 and another 20% were infected by HPV-18.7 By that time, these and other high-risk types of HPV were also recognized as important causes of cancers of the head and neck, such as throat or oropharynx (OPSCC).8 The International Agency for Research on Cancer (IARC) regards the following HPV genotypes as Group 1 carcinogens (cancer causing): HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52, HPV-56, HPV-58, HPV-59, and HPV-66.8
In most cases, our body’s immune system will clear HPV just like any other infection. However, if an infection with a high-risk type of HPV lasts for 10 years or more, the infected tissue can eventually become malignant (cancerous).9 These lesions are easiest to treat when they are found early — before they become malignant or before the malignancy has spread to other organs in the body (metastasized). Early detection is the purpose of annual screening with the Papanicolaou (Pap) test for cervical cancer.10 Unfortunately, screening tests do not exist for other cancers commonly caused by HPV, like head and neck or anal cancers. Thus, physicians need a biomarker to help detect and monitor those cancers.

Tumor tissue modified viral (TTMV®)-HPV DNA is a biomarker found in patients with cancers driven by human papillomavirus (HPV), making it an ideal biomarker. Routine NavDx testing to establish and track changes in a patient’s TTMV Score can offer a more complete picture of cancer status than physical exams and imaging scans provide.
The NavDx blood test is currently being used to help physicians detect and monitor HPV+ oropharyngeal (head and neck) and anal cancer. NavDx testing is used to confirm that the tumor is HPV-positive, to assess treatment response, and to detect recurrences, especially in distant sites. The test is also being studied for use in cases of HPV-driven cervical cancer.
The rates of squamous cell carcinoma of the oropharynx (the lining of the mouth and throat) has been increasing in the U.S. and worldwide.11 The cause of this growth in oropharyngeal squamous cell carcinoma (OPSCC) is an increase in oral infections with high-risk HPV, especially HPV-16.11 In fact, HPV-driven head and neck cancer now represents 60% to 70% of all head and neck cancer in the United States.11
HPV-positive oropharyngeal cancers tend to occur in younger people, often without other risk factors such as cigarette smoking and alcohol abuse.12 These tumors appear initially in the oropharynx and tend to be small, but with high involvement of lymph nodes.12 Most importantly, people diagnosed with HPV-positive head and neck cancer tend to have a better prognosis (outcome), and respond better to treatment than those diagnosed with tobacco-related, HPV-negative OPSCC. HPV-positive OPSCC, such as throat cancer, typically respond very favorably to radiation or chemotherapy, with cure rates ranging between 65% and 95%—as opposed to a 30-45% cure rate for tobacco-related head and neck cancer.13 The overall survival rate is also higher for those with HPV-positive oropharyngeal cancer than those who are HPV-negative; five-year overall survival rates for patients with advanced-stage HPV-positive OPSCCs are approximately 75% to 80%.14
Tumors of the oropharynx (mouth and throat) can affect many daily activities, such as chewing, swallowing, breathing, speaking, and sensation. These tumors may also have a negative effect on cosmetic appearance.15 Fear of recurrence is the single largest patient concern after treatment.16 Survivors of this cancer have demonstrated high rates of psychological distress and compromised quality of life.16 Even with successful treatment, 13%-25% of patients with HPV-driven head and neck cancer will experience a recurrence within 5-years.14,17,18 Thirty-three to 52% of first recurrences are concerning distant metastases.18,19
While imaging has an important role in monitoring for recurrence, it also has clear limitations in terms of sensitivity, and ability to detect occult (distant) metastases outside the region being imaged.20 Physicians need a biomarker that will let them assess treatment response and detect recurrences earlier than it would present clinically.
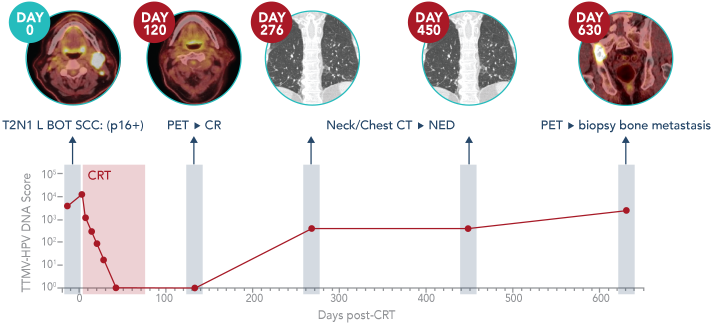
Tumor tissue modified viral (TTMV)-HPV DNA is a unique cancer biomarker that tumor cells of cancers driven by human papillomavirus shed into the blood.21 After entering cells, HPV DNA can integrate in the cellular genome and become linked to human DNA sequences or remain episomal.22 As these tumor cells die, they release TTMV-HPV DNA into the blood. Circulating TTMV-HPV DNA is found in people with HPV-driven cancers and is distinct from noncancerous HPV DNA, making it an ideal biomarker.20 Routine testing to track changes in a person's circulating blood TTMV Score can offer a more complete picture of cancer status than relying on physical exams and imaging scans.20

The NavDx test is the first and only clinically validated circulating TTMV-HPV DNA blood test that can aid in the detection and monitoring of HPV-driven cancer. Routine NavDx testing before, during, and at defined intervals after treatment enables physicians to optimize the clinical management of HPV-positive head and neck or anal cancer and reassure patients that their disease is being effectively treated and monitored.20
References
1. HPV infection. Updated October 12. Accessed January 17, 2022.
https://www.mayoclinic.org/diseases-conditions/hpv-infection/symptoms-causes/syc-20351596.
2. Chen Z, Schiffman M, Herrero R, et al. Classification and evolution of human
papillomavirus genome variants: Alpha-5 (HPV26, 51, 69, 82), Alpha-6 (HPV30, 53, 56, 66), Alpha-11
(HPV34, 73), Alpha-13 (HPV54) and Alpha-3 (HPV61). Virology. Mar 2018;516:86-101.
doi:10.1016/j.virol.2018.01.002. 3. Steele K, Shirodaria PV, Pfister H, et al. A
study of HPV 1, 2 and 4 antibody prevalence in patients presenting for treatment with cutaneous warts
to general practitioners in N. Ireland. Epidemiol Infect. Dec 1988;101(3):537-46.
doi:10.1017/s0950268800029411. 4. Division of STD Prevention, National Center for
HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention. Genital HPV
infection – fact sheet. Centers for Disease Control and Prevention. Accessed January 5, 2022.
https://www.cdc.gov/std/hpv/std-fact-hpv.htm. 5. Human papillomaviruses. IARC
Monographs. 2012;100B:255-313. 6. American Cancer Society. HPV and cancer. Updated
July 30, 2020. Accessed January 17, 2022.
https://www.cancer.org/cancer/cancer-causes/infectious-agents /hpv/hpv-and-cancer-info.html.
7. Zur Hausen H. Nobel lecture.
https://www.nobelprize.org/prizes/medicine/2008/hausen/lecture/. 8. IARC Working
Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, no. 90. Vol. 90. 2007. https://www.ncbi.nlm.nih.gov/books/NBK321760/.
9. Ratini M. Oral HPV and cancer. WebMD.
https://www.webmd.com/oral-health/guide/oral-hpv-cancer. Updated January 20, 2022. Accessed March 14,
2023. 10. American Cancer Society. The Pap (Papanicolaou) test. Updated January 3.
Accessed January 17, 2022.
https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/screening-tests/pap-test.html.
11. Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption
in 187 countries, 1980-2012. JAMA. Jan 8 2014;311(2):183-92. doi:10.1001/jama.2013.284692.
11. Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in
incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. Dec 20 2013;31(36):4550-9.
doi:10.1200/JCO.2013.50.3870. 12. Kostareli E, Holzinger D, Hess J. New concepts for
translational head and neck oncology: lessons from HPV-related oropharyngeal squamous cell carcinomas.
Front Oncol. 2012;2:36. doi:10.3389/fonc.2012.00036. 13. Posner M, Genden E. Cell
Free HPV DNA: A new diagnostic and predictive test for HPV oropharynx cance. News from SPOHNC2021. p.
1-3. 14. Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human
papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl
Cancer Inst. Feb 20 2008;100(4):261-9. doi:10.1093/jnci/djn011. 15. Cancer Research
UK. Changes in your appearance. Updated October 14, 2021. Accessed January 14, 2022.
https://www.cancerresearchuk.org/about-cancer/mouth-cancer/living-with/changes-your-appearance.
16. Elaldi R, Roussel LM, Gal J, et al. Correlations between long-term quality of
life and patient needs and concerns following head and neck cancer treatment and the impact of
psychological distress. A multicentric cross-sectional study. Eur Arch Otorhinolaryngol. Jul
2021;278(7):2437-2445. doi:10.1007/s00405-020-06326-8. 17. Ang KK, Harris J, Wheeler
R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. Jul 1
2010;363(1):24-35. doi:10.1056/NEJMoa0912217. 18. Gillison ML, Trotti AM, Harris J,
et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer
(NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. Jan 5
2019;393(10166):40-50. doi:10.1016/S0140-6736(18)32779-X. 19. Fakhry C, Zhang Q,
Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal
squamous cell carcinoma. J Clin Oncol. Oct 20 2014;32(30):3365-73. doi:10.1200/JCO.2014.55.1937.
20. Chera BS, Kumar S, Shen C, et al. Plasma circulating tumor HPV DNA for the
surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J Clin Oncol. Apr 1
2020;38(10):1050-1058. doi:10.1200/JCO.19.02444. 21. Chera BS, Kumar S, Beaty BT, et
al. Rapid clearance profile of plasma circulating tumor HPV type 16 DNA during chemoradiotherapy
correlates with disease control in HPV-associated oropharyngeal cancer. Clin Cancer Res. Aug 1
2019;25(15):4682-4690. doi:10.1158/1078-0432.CCR-19-0211. 22. Pappa KI, Kontostathi
G, Lygirou V, Zoidakis J, Anagnou NP. Novel structural approaches concerning HPV proteins: Insight
into targeted therapies for cervical cancer (Review). Oncol Rep. Apr 2018;39(4):1547-1554.
doi:10.3892/or.2018.6257.
about how the NavDx test is helping
optimize HPV-driven cancer care
This site is protected by reCAPTCHA and the Google Privacy
Policy.
Terms of Service apply.
about how the NavDx test is helping
optimize HPV-driven cancer care
This site is protected by reCAPTCHA and the Google Privacy Policy.
Terms of Service apply.